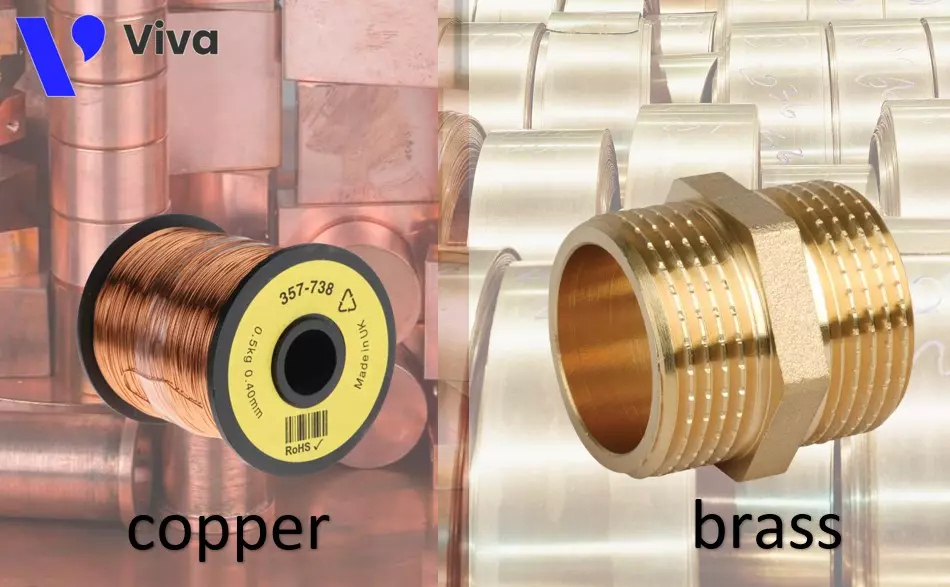
Brass
Brass alloys are important materials used in various fields, and they are used to manufacture many devices and products that serve humans. In this article, let’s explore useful information related to this type of material, including: What is a copper alloy? Its characteristics and properties, practical applications, formation, and development…
Now, we invite our readers to the main content of the article!
Learn about brass
What is brass?
Brass is a type of alloy composed mainly of copper and zinc, with copper ranging from 55% to 95% and zinc ranging from 5% to 45%. Depending on the material requirements, other elements such as aluminum, nickel, and lead may be added during the manufacturing process.
This alloy is known for its high strength, ease of machining, corrosion resistance, and good impact resistance.
Based on the proportion of different components in the material, brass is classified into various types with different properties.
Depending on the specific characteristics of each product, brass is applied in various fields, including mechanical engineering, interior decoration, musical instruments, and more.

How is brass produced?
The production process of brass starts with preparation, where the raw materials including copper, zinc, and other additives are gathered.
The copper and zinc are then cut into small pieces, placed in a furnace, and heated to their melting point. When all the material components have turned into a liquid state, they are stirred together to achieve a homogeneous copper solution. The additives play a role in enhancing bonding and removing unwanted impurities during the manufacturing process.
The molten brass is then poured into molds to cool and solidify, taking the desired shape. After the brass has cooled and hardened, it can undergo additional processing, such as polishing or mechanical machining, to achieve the desired finish or shape.

When was bronze first used?
Bronze was discovered for the first time around 500 BCE and is a material that has a color similar to “pure gold.”
Ancient Romans used bronze to create decorative objects and jewelry because this material has a metallic luster and is resistant to oxidation under normal conditions.
It was used to make coins and weapons due to its hardness, high durability, and corrosion resistance.
During the Middle Ages, bronze was used to manufacture various devices and objects such as components in telescopes. Bronze was polished to make mirrors and various other objects.
During the Industrial Revolution, starting from the late 18th century, bronze was used more extensively to produce machine components.
Properties of bronze
Like all other materials, bronze also has its own unique properties, and when studying bronze or any other material, understanding the properties of the material is always important. It allows us to determine which material is suitable for specific tasks.
- Color: Bronze typically has a golden metallic color.
- Thermal and electrical conductivity: Bronze has relatively good thermal and electrical conductivity.
- Melting point: It ranges from 900°C to 940°C (depending on the composition).
- Strength: Bronze has relatively high strength, as it possesses mechanical and chemical properties including corrosion resistance, hardness, ductility, and the ability to be thinly coated. These properties give this material the ability to withstand impact, work with certain chemicals, and withstand relatively high temperatures.
- Average density: It ranges from 8.3 g/cm³ to 8.7 g/cm³ (depending on the composition).
- Easy to process: Bronze is considered a material that can be easily processed. It can be shaped into various objects or details through different processing methods such as casting, turning, milling, planing, forging, and stamping.

Applications of Brass in Industry and Daily Life
Starting from the unique properties of brass and going through a long process of practical application, this material is used to manufacture many different types of objects and components.
Some examples of practical applications of brass include:
- In daily life: Around us, we can see many objects made of brass, including interior decorations, water pipes, and various household items.
- In machinery: This material is widely used in various types of machinery to manufacture components and mechanisms.
- In electronic devices: In addition to mechanical machines, brass is commonly used in electronic devices. With its good corrosion resistance and electrical conductivity, this material is often used for electrical conduction purposes.
- Used in the production of valves and pipe fittings: Flanged brass ball valves, brass gate valves, brass ball valves, and more.
These are just a few examples that demonstrate the application potential of brass, and of course, it does not stop there. This material is used in many other fields as well. Through the examples above, it is hoped that readers can gain a better understanding of the properties and importance of brass.

Strengths and Limitations of Brass
As a widely used material in various applications, brass is generally considered to have many advantages over other materials. However, along with that, this material still has certain limitations. What are they? Let’s find out together.
Advantages
- High corrosion resistance: It has the ability to resist corrosion and oxidation, making it commonly used in applications that require durability and high strength.
- Easy to process: Due to its malleability and ease of cutting, drilling, bending, and welding, it is easy to process into various parts.
- High durability: It has good strength and impact resistance, making it a preferred material in manufacturing various machine parts and equipment.
- Good electrical and thermal conductivity: It has good electrical and thermal conductivity, making it often used in electronic applications and cooling systems.
Limitations
- High cost: Copper is a material with many desirable properties, but it requires multiple processes to create metal blanks. Therefore, compared to many other materials, copper has a relatively high price when considering the same weight of material.
- Corrosion when exposed to strong corrosive chemicals: Although copper has corrosion resistance, it can still corrode when directly exposed to highly corrosive chemicals. This is an important consideration when selecting materials for design and manufacturing.
- Low heat resistance: Copper has a melting temperature range of 900°C to 940°C, which is not considered high in terms of heat resistance.
Considering the advantages and disadvantages mentioned above, copper is generally a material with many good properties, suitable for manufacturing various parts, equipment, and tools.
Comparison between Copper Alloy and Pure Copper
Both copper alloy and pure copper are materials that have been used for a long time and are still widely used today. They have different origins, so they are used in many different applications. However, they also have many similarities, so in practice, some people still have some confusion about these two materials. The following content we provide aims to help readers understand these two materials better.
- Color: Copper alloy has a bright yellow color, while pure copper has a reddish-brown color.
- Chemical composition: Pure copper consists of 100% copper (Cu), while copper alloy is a type of alloy composed mainly of copper and zinc, along with other additional elements. The percentage composition of these elements in copper alloy can vary depending on the purpose.
- Mechanical properties: Due to the inclusion of zinc and other components, copper alloy is harder than pure copper. However, pure copper has higher tensile strength.
- Thermal and electrical conductivity: Copper has higher conductivity in both heat and electricity compared to copper alloy.
- Applications: Although both copper and copper alloy are frequently used in many related applications, their distinct physical and chemical properties determine their specific uses. Copper is commonly used for conducting electricity and heat, while copper alloy is used for applications that require higher strength.